Abstract

Introduction: In 2019, international guidelines for the management of immune thrombocytopenia (ITP) stated that ITP treatment is rarely indicated in case of platelet count >20 x109/L. The threshold associated with significant bleeding in ITP patients treated with antiplatelet agents is unknown, and 2019 American society of hematology guidelines for ITP management stressed the need for studies to determine it. This was the aim of this study.
Methods: The source of data was the CARMEN-France registry, a prospective, real-world registry of incident ITP adults in the French Midi-Pyrénées region since June 2013, implemented in other centers of the French Autoimmune cytopenia referral center network since 2016. Inclusion criteria were adult patients (aged ≥18 years) newly diagnosed with ITP (platelet count <100 x 109/L and exclusion of other causes of thrombocytopenia). We designed a transversal study to correlate the platelet count to bleeding at ITP diagnosis, before any ITP treatment. Study population consisted in the patients included in the registry up to November 2021 and treated with antiplatelet agents at the time of ITP diagnosis, without concomitant exposure to anticoagulant. We quantified the percentages of patients with overall bleeding, cutaneous bleeding, non-serious mucosal bleeding and serious bleeding (defined by intracranial bleeding, gastro-intestinal bleeding or gross hematuria with anemia) by categories of platelet count (by 109/L up to 50 x 109/L then ≥50 x 109/L). Analyses were conducted by subgroups of antiplatelet agents due to suspected differences in pharmacological effect in patients with ITP who have a high platelet turn over: patients treated with clopidogrel and ticagrelor, two reversible ligands to P2Y12 receptor with longer half-life, may be at a higher risk of bleeding than those treated with acetylsalicylic acid alone, an irreversible ligand to COX-1.
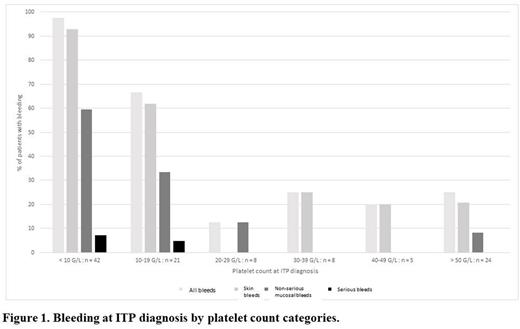
Results: Out of 1171 adult patients with incident primary ITP included in the registry during the study period, 143 (12.2%) were exposed to an antiplatelet drug at the time of ITP diagnosis: 108 to acetylsalicylic acid alone, 20 to clopidogrel alone and 15 to an association of antiplatelet agents (acetylsalicylic acid + clopidogrel, n=13; acetylsalicylic acid + ticagrelor, n=2). In the acetylsalicylic acid group, the median age was 75.5 years, 73 (67.6%) were men, median platelet count at ITP diagnosis was 15 x 109/L and 66 (61.1%) had bleeding: 60 (55.6%) a cutaneous bleeding, 35 (32.4%) a non-serious mucosal bleeding and 4 (3.7%) a serious bleeding (3 gastrointestinal bleeding with anemia and 1 gross hematuria with anemia). The pattern of bleeding by platelet count categories is shown in Figure 1. The frequency of overall bleeding was 97.6% (41/42) in the < 10 x 109/L group, 66.7% (14/21) in the 10 - 19 x 109/L group and 22.2% (10/45) in the ≥20 x 109/L group (similar pattern by all categories ≥20 x 109/L). The receiver operating characteristics curve had an area under the curve of 88.3% (95% confidence interval: 81.9-94.6%). The threshold of <20 x 109/L had a sensitivity of 83.3% and a specificity of 81.0%. The pattern for cutaneous and non-serious mucosal bleeding was similar (Figure 1). All four serious bleeding occurred in patients with a platelet count <20 x 109/L. In the clopidogrel and association of antiplatelet drug groups, bleeding was observed in 14 (70.0%) and 10 (66.7%) patients, respectively. No clear correlation with the platelet count was shown, limited by the low number of patients. One serious bleeding occurred in each group (two intracranial bleeding), in patients with platelet counts < 10 x 109/L.
Conclusion: This study shows that the bleeding risk in ITP patients treated with acetylsalicylic acid alone is similar to other ITP patients.
Disclosures
Piel-Julian:Novonordisk: Other: Speaker at educational session. Viallard:Novartis: Other: Boards, speaker at educational sessions; Grifols: Other: Boards, speaker at educational sessions; Amgen: Other: Boards, speaker at educational sessions. Comont:AbbVie: Other: Boards, speaker at educational sessions; AstraZeneca: Other: Boards, speaker at educational sessions; Celgene: Other: Boards, speaker at educational sessions; Takeda: Other: Boards, speaker at educational sessions; Novartis: Other: Boards, speaker at educational sessions. Chèze:Novartis: Other: Board; Protalex: Research Funding; Novartis: Research Funding; Bioverativ: Research Funding; Sobi: Other: Board. Audia:grifols: Other: Speaker at educational sessions. Ebbo:Novartis: Other: Boards, speaker at educational sessions; Grifols: Other: Boards, speaker at educational sessions; Amgen: Other: Speaker at educational sessions. Terriou:Amgen: Other: Board; Grifols: Other: Board; Novartis: Other: Board. Bonnotte:Novartis: Other: Speaker at educational sessions. Michel:UCB: Other: Boards, speaker at educational sessions; argenx: Other: Boards, speaker at educational sessions; Novartis: Other: Boards, speaker at educational sessions; Sobi: Other: Boards, speaker at educational sessions; Amgen: Other: Boards, speaker at educational sessions. Godeau:Grifols: Other: Boards, speaker at educational sessions; Sobi: Other: Boards, speaker at educational sessions; Novartis: Other: Boards, speaker at educational sessions; Amgen: Other: Boards, speaker at educational sessions. Moulis:Amgen: Other: Boards, speaker at educational sessions; Argenx: Other: Board; Grifols: Other: Boards, speaker at educational sessions, Research Funding; Novartis: Other: Boards, speaker at educational sessions, Research Funding; Sobi: Other: Board.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal